Repetitive Transcranial Magnetic Stimulation was approved by the FDA in October 2008 for patients with major depression who have failed one prior antidepressant trial.
Stimulation of the brain is accomplished by a pulsed magnetic field that is passed through a coil of wire encased in plastic and held close to the head. This magnetic field penetrates the scalp and skull. The stimulation is made at regular intervals, thus the term “repetitive” TMS.
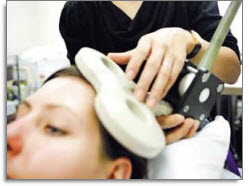 In studies, rTMS appears to change brain activity beyond the duration of the actual procedure. Also, the procedure differs from Electroconvulsive Treatment (ECT) in that it stimulates the brain in a focal manner, thereby preventing the grand mal seizure and minimizing the transitory memory loss associated with ECT.
In studies, rTMS appears to change brain activity beyond the duration of the actual procedure. Also, the procedure differs from Electroconvulsive Treatment (ECT) in that it stimulates the brain in a focal manner, thereby preventing the grand mal seizure and minimizing the transitory memory loss associated with ECT.
rTMS is performed on an outpatient basis, with a course of 20-30 treatments, each lasting approximately 40 minutes, and delivering 3,000 pulses. The most common reported side effect is mild headache. The cost per 40 minute session: approximately $400. Ouch!





Leave A Comment
You must be logged in to post a comment.